Review Process
HOW IT WORKS
- Protocols are reviewed by either designated or full committee review.
- Designated reviews are completed by at least two committee members and a veterinarian.
- Full committee review involves primary and secondary reviewers presenting to the full committee at a convened committee meeting.
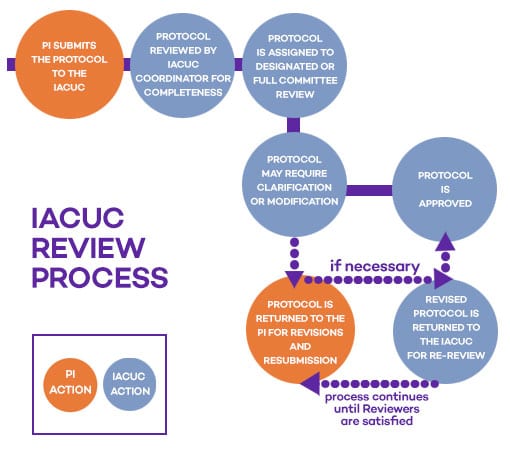 Committee members receive all submissions. The Chair/designee appoints a small subset of members as designated reviewers of each submission. Most submissions fall under designated review, which does not have a submission deadline. Some submissions are designated for full committee review (FCR), and require review at a convened meeting of the IACUC. Protocols describing work with primates, severe food and/or water restriction of any species and other special review items (as defined in the policy on IACUC Review of Animal Study Protocols, Addenda Requests, and Annual Certifications) are automatically sent for FCR. To ensure FCR at a particular meeting, protocols must be submitted 10 days before a scheduled meeting.
Committee members receive all submissions. The Chair/designee appoints a small subset of members as designated reviewers of each submission. Most submissions fall under designated review, which does not have a submission deadline. Some submissions are designated for full committee review (FCR), and require review at a convened meeting of the IACUC. Protocols describing work with primates, severe food and/or water restriction of any species and other special review items (as defined in the policy on IACUC Review of Animal Study Protocols, Addenda Requests, and Annual Certifications) are automatically sent for FCR. To ensure FCR at a particular meeting, protocols must be submitted 10 days before a scheduled meeting.
The Department of Defense (DoD) requires the annual review (prior to the beginning of years 2 and 3) of all DoD-funded protocols involving animals covered by the United States Department of Agriculture (USDA). In accordance with this, Northwestern policy requires all protocols listing covered species be reviewed/certified annually if they are funded by the DoD. All other protocols are fully re-reviewed every 3 years.
APPROVAL
Approval means a project may begin. The PI may order animals from the Center for Comparative Medicine (CCM). Project personnel may gain access to the animal facilities. The PI may talk to Sponsored Research (SR) about opening accounts to spend grant awards.
Details about approval:
- The IACUC approves protocols for a three-year period, even if sponsored funding is for a longer term.
- DoD-funded protocols involving work with USDA covered species must be submitted for annual re-certification.
- A de novo (new) protocol must be submitted to the IACUC prior to the end of the initial three-year approval period to avoid inactivation.